Testing of Prefilled Syringes to ISO 11040
A prefilled syringe (PFS) is a disposable needle-based injection system pre-filled with a specified substance to be administered. Many autoinjectors rely on a prefilled glass syringe for drug containment and delivery. This prefilled syringe consists of various components, which must be tested using a variety of standard tests to confirm the integrity and functionality of the syringe system, and ultimately ensure proper containment and expulsion of the drug and the highest possible level of comfort for the patient.
Definition prefilled syringe Description ISO 11040 Video ISO 11040-4/-8 ISO 11040-4 tests C1 C2 E F G1 G2 G3 G4 G5 G6 Testing systems for syringes Downloads
Definition of prefilled syringe
Types of prefilled syringes (PFS) include glass or plastic, with or without needle:
- Glass prefilled syringe: this material represents the highest level of experience in the industry, and still makes up the majority of prefilled syringe packaging today.
- Plastic prefilled syringe (polymer): the use of plastic syringes is growing due to advancements in polymer materials that have increased their biocompatibility with other syringe components.
- Needle-free prefilled syringe: these syringes are commonly used for vaccine products and include a Luer lock attachment for the needle, some type of tamper-proof element, and an elastomer plug at the nozzle of the syringe.
- Pre-filled syringe with staked-in needle: this type of syringe is typically used with biologic and pharmaceutical drugs and has the needle pre-attached (adhered) to the syringe.
As is the case with any tool used in the medical and pharmaceutical industry, the PFS is designed to meet the needs of the user, while maintaining strict standards of quality, efficacy, and safety. When compared to the traditional vial-syringe format, which is still widely used today, the PFS offers various advantages:
- Decreased contamination possibilities
- Decreased tendency to overfill
- Increased dosing accuracy
- Increased convenience
- Increased safety features
Along with, in some cases life-saving benefits, the PFS bears a multi-component complexity that includes a barrel, a needle, a needle shield, a plunger and a closure, all of which must interact seamlessly and safely with each other and with the pre-filled drug. In addition, the container closure integrity ( CCI), that is, the suitability of the syringe closure system, is of critical importance to maintain a sterile barrier against potential contaminants. Every version of the PFS is therefore strictly regulated by international standards and subject to testing.
Syringe testing to ISO 11040 – prefilled syringes
The ISO 11040 standard consists of eight parts under the general title Prefilled Syringes, each of which covers different PFS components. Syringe testing to ISO 11040 is critical for safe dispensing and administration of medications, anticoagulants, and biologics. To meet the strict requirements of the annexes included in the standard, ZwickRoell offers the right syringe testing equipment for your needs.
- Our testing machines can be adapted with a range of interchangeable test fixtures that accommodate a multitude of syringe types and are designed for standardized prefilled syringe testing to ISO 11040 (ISO 11040-4, ISO 11040-6, ISO 11040-8), also referenced in USP (United States Pharmacopeia) Chapters <1382> and <382>.
- With a traceability option, our ZwickRoell testing software meets the requirements necessary for compliance with all FDA 21 CFR Part 11 criteria.
Syringe testing to ISO 11040 helps determine the integrity and functionality of the syringe:
- ISO 11040-4: Glass barrels for injectables and sterilized subassembled syringes ready for filling (ISO 11040-4:2015)
- ISO 11040-6: Plastic barrels for injectables and sterilized subassembled syringes ready for filling
- ISO 11040-8: Requirements and test methods for finished prefilled syringes
ISO 11040-4 Mechanical testing of glass barrels for injectables
At ZwickRoell we provide a few, purposefully designed, easy-to-use and standard-compliant tools for the performance of each one of these tests.
Based on the needs and feedback from many of our customers, we have designed, developed, and built a few standard-compliant test fixtures and tools to ensure an outstanding level of efficiency and absolute reliability for a wide variety of tests. All test fixtures are seamlessly compatible with every ZwickRoell testing machine.
In addition to a universal testing machine for tensile, compression and torsion testing, the respective load cells, and our intuitive testXpert testing software, we offer the right tools to ensure:
- Efficiency - With only a few tools, you can quickly and easily change out specimens and test setups, saving both time and money.
- Repeatability - The right alignment aids ensure uncomplicated, accurate placement of your specimens to ensure identical conditions for successive measurements.
- Reliable test results - Seamless interaction and compatibility of our testing system, standard-compliant test fixtures and tools, and our testXpert testing software with both standard and customizable test programs guarantee reliable test results.
The ISO 11040-4 standard specifies 10 different tests for prefilled glass syringes
Needle shield
G6: Pull-off force of the tip cap or needle shield
G2: Closure system liquid leakage test
Plunger stopper
E: Glide force test method to evaluate syringe lubrication
Round flange
C1: Flange breakage resistance
Needle
F: Needle penetration test
G1: Needle pull-out force
Luer cone
C2: Luer cone breakage resistance
Adapter collar
G3: Luer lock adapter collar pull-off force
G4: Luer lock adapter collar torque resistance
Tip cap
G5: Luer lock rigid tip cap unscrewing torque
Annex C1: Flange breakage resistance
Upon actuation, an abrupt, high initial force is generated in an autoinjector that can break the round flange at the base of the syringe barrel and prevent proper delivery of the intended drug. The flange breakage resistance test measures the force at which this flange breaks and can be performed using our standard-compliant accessories.
1 Universal syringe holder
2 Quick-closing handle
3 Alignment aid (plastic disc)
4 Lower support surface
Universal syringe holder
One fixture with four fingers designed in a cross pattern, that move in and out to easily accommodate different types and sizes of syringes in the vertical position. The required distance between the fingers is set according to the syringe diameter to prevent force application on the syringe. The syringe is placed vertically in the center of the fingers, which are then closed manually with a quick-closing handle, gripping the syringe. To open the fingers and remove the syringe after the test, the handle is turned to the other side, and the fixture is ready to receive the next specimen.
Alignment aid (plastic disc)
Plastic disc to guide and straighten the syringe in the test fixture without applying lateral forces that could alter the test results. The disc has various size holes to accommodate different syringe diameters, it is made of a standard-compliant material and enables homogeneous force application on the flange.
Lower support surface
A support surface below the syringe holder designed to hold a beaker (included with fixture) for collection of expelled fluids and an optional scale (accessory) to weigh the expelled fluid and ensure that the syringe has fully emptied.
Benefits:
- One fixture for a variety of syringe diameters
- Easy to use
- Precise specimen alignment
- Convenient fluid collection
Annex C2: Luer cone breakage resistance
The Luer cone presents a weak point on the syringe assembly. Lateral forces can cause the cone to break, preventing delivery of the intended drug and rendering the device useless. The Luer cone breakage resistance test measures the maximum force at which the cone breaks.
For this test, the standard specifies that a die be used to apply a compressive force at a precise location on the cone. It is therefore crucial that the syringe is placed in the exact alignment position with relation to the die. Our test fixture for horizontal placement of the specimen is designed for easy insertion of the syringe in the exact required location, without the need to first remove the needle.
1 Hinged specimen grip
2 Syringe adapter
3 Manual lever
4 Alignment aid (metal plate)
5 Space for needle
6 Alignment aid recess
7 Flip-top safety cover
8 Collection tray
Syringe adapter
Plastic adapter specific for different sizes and diameters of syringes. The syringe is placed inside the plastic adapter before it is inserted in the specimen grip. To save time when testing multiple specimens, each additional syringe can be placed in an adapter ahead of time and can then be inserted in the jaws after each tested specimen is removed. No parts or components need to be screwed on or off when changing syringe sizes.
Hinged specimen grip
The syringe in the adapter is inserted horizontally into the lower clamping jaw. The upper jaw is flipped down over the syringe A manual lever is now folded upwards to lock it in place.
Alignment aid (metal plate)
The metal alignment plate is moved towards the handle by manually pushing it as far as it will go. The recess in the alignment aid provides the precise length at which the cone must protrude from the grip for alignment with the die. The alignment aid has a space for the needle, eliminating the need to remove it prior to testing.
Flip-top safety cover
The attached safety cover is flipped over the test area before starting the test to prevent possible injury from ejecting pieces of broken glass.
Collection tray
The tray allows for safe removal of broken syringe fragments.
Benefits:
- Easy to use
- Precise specimen alignment
- No need to remove the needle
Annex E: Glide force test method to evaluate syringe lubrication
Proper glide force of the syringe plunger is imperative for continuous delivery of the intended drug. One determining factor of the glide force is the condition of the oil lubricant that lines the inside of the syringe barrel. If the barrel is not properly lubricated, or the silicone oil has begun to dry throughout the shelf-life of the injector—a condition that can be simulated for testing purposes—the plunger may experience an initial break-loose force and become unable to glide at the speed and with the continuity the standard requires. While Annex E of the ISO 11040-4 standard describes the glide force test, here you can also opt to test the break-loose force by configuring your testXpert testing software accordingly.
1 Universal syringe holder
2 Lower support surface
3 Concave die
4 Quick-closing handle
Universal syringe holder
One fixture with four fingers designed in a cross pattern, that move in and out to easily accommodate different types and sizes of syringes in the vertical position. The required distance between the fingers is set according to the syringe diameter to prevent force application on the syringe. The syringe is placed vertically in the center of the fingers, which are then closed manually with a quick-closing handle, gripping the syringe. To open the fingers and remove the syringe after the test, the handle is turned to the other side, and the fixture is ready to receive the next specimen.
Concave die
As the die is moved in the compressive direction to push down the plunger, the center of the curvature of the concave die lines up with the top end of the plunger and aligns and steadies the plunger as it is pressed down into the syringe barrel. The inner curvature of the die eliminates any movement or jumping motion of the plunger upon making contact with the die, thereby preventing unwanted influences on the test result.
Lower support surface
Designed to hold a beaker (included with fixture) for collection of expelled fluids and an optional scale (accessory) to weigh the expelled fluid and ensure that the syringe has fully emptied.
Benefits:
- One fixture for a variety of syringe diameters
- Easy to use
- Precise specimen alignment
- Steady positioning of the syringe
- Convenient fluid collection
Annex F: Needle penetration test
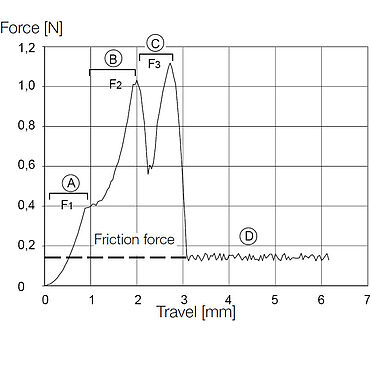
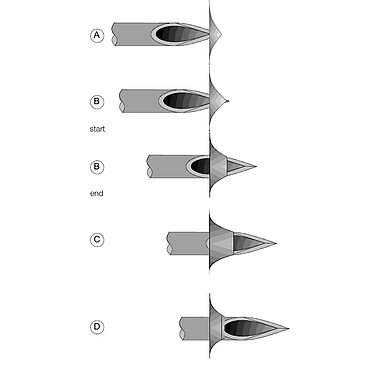
The needle tip, or bevel of the needle on a syringe is fairly complex and must adhere to strict standards and specifications. Measurements performed with the needle penetration test are used to characterize the point sharpness of the needle, the frictional force along the shaft of the needle, and the effectiveness of the needle tip geometry. These characteristics ultimately influence the comfort level of the patient and the trauma caused to the injection site—the higher the force required for penetration, the higher the level of discomfort. This test method is used to determine the needle penetration force by piercing a test foil that mimics human skin with a needle.
1 Pneumatic grip
2 Test foil clamping fixture
Pneumatic grip
Pneumatic grip with special jaws that apply even pressure on the syringe barrel using the lowest amount of force possible to prevent breakage as it is moved downward in the compressive direction and the needle penetrates the foil.
Test foil clamping fixture
a special fixture to securely hold the foil without applying tension. While the standard only requires the needle to penetrate the foil in a perpendicular position, our clamping fixture was designed with the option to be tilted. When used on a patient, syringes are typically held and inserted at an angle. This angle affects the force required to penetrate the skin. In terms of syringe testing to ISO 11040-4, the tilt feature is simply an option, not a requirement.
Different types of test foil can be used, for example, in accordance with DIN 13097-4 or ISO 7864. The most commonly used foil is made of polyurethane with a thickness of 0.4 mm.
Advantages:
- Easy to use
- Precise specimen alignment
- Force measurement at angled needle penetration
Annex G1: Needle pull-out force (needle bond strength)
The needle on a syringe must be able to withstand the force required to pull the needle out without detaching from the syringe assembly. Insufficient needle pull-out forces can be the result of weak needle assemblies or imperfect needle adhesion. For this test, the syringe is secured vertically in the test fixture with the needle facing up, the needle is clamped with special jaws to prevent slippage, and a tensile force is applied to pull the needle upward.
1 Pneumatic specimen grip (upper)
2 Pneumatic specimen grip (lower)
3 Wave-pattern jaws
Pneumatic specimen grip (lower)
Pneumatic specimen grip with special jaws that apply even pressure on the syringe barrel using the lowest amount of force possible to prevent breakage.
Pneumatic specimen grip (upper)
Pneumatic grip with wave-pattern jaws that securely hold the needle. This specific jaw pattern holds the needle securely with the least amount of pressure, when compared to other types of jaws, keeping the needle from being crushed while also preventing slippage that could have unwanted effects on the test results.
Advantages:
- Easy to use
- Precise specimen alignment
- No needle slippage
Annex G2: Closure system liquid leakage test
Prefilled syringes require a standard-compliant closure system for contamination prevention, storage, and transportation purposes. This test is designed to verify whether the closure system is able to withstand any potential overpressure inside the syringe during the filling process or during transportation. The test setup and procedure are the same as the glide force test, except in this case, the syringe is closed with a tip cap and the result is recorded as pass/fail. A specified amount of force is applied for a predetermined duration. The test is passed if the tip cap does not come off and/or if no droplets are visible around the external surfaces of the closure system.
1 Universal syringe holder
2 Lower support surface
3 Concave die
4 Quick-closing handle
Universal syringe holder
One fixture with four fingers designed in a cross pattern, that move in and out to easily accommodate different types and sizes of syringes in the vertical position. The required distance between the fingers is set according to the syringe diameter to prevent force application on the syringe. The syringe is placed vertically in the center of the fingers, which are then closed manually with a quick-closing handle, gripping the syringe. To open the fingers and remove the syringe after the test, the handle is turned to the other side, and the fixture is ready to receive the next specimen.
Concave die
As the die is moved in the compressive direction to push down the plunger, the center of the curvature of the concave die lines up with the top end of the plunger and aligns and steadies the plunger as it is pressed down into the syringe barrel. The inner curvature of the die eliminates any movement or jumping motion of the plunger upon making contact with the die, thereby preventing unwanted influences on the test result.
Lower support surface
Designed to hold a beaker (included with fixture) for collection of expelled fluids and an optional scale (accessory) to weigh the expelled fluid and ensure that the syringe has fully emptied.
Benefits:
- One fixture for a variety of syringe diameters
- Easy to use
- Precise specimen alignment
- Steady positioning of the syringe
- Convenient fluid collection
Annex G3: Luer lock adapter collar pull-off force
Luer fittings are designed to provide leak-free connections between syringes and needles or tubes (e.g., IV tubes). The Luer lock collar conforms to the Luer cone dimensions and is pressed onto the cone and held by friction. The Luer lock collar pull-off force test is designed to verify whether the collar is able to withstand an axial pull-off force and avoid premature detachment from the syringe barrel. Premature detachment would render the syringe system useless. For the test, the syringe barrel is vertically secured in a pneumatic grip with the tip facing upward. The Luer lock collar is then pulled upward until it detaches from the Luer cone. Depending on whether it sustained the required amount of force before detaching, a determination is made of whether the specimen passed or failed.
1 Special self-aligning specimen grip
2 Pneumatic specimen grip (lower)
Pneumatic specimen grip (lower)
Pneumatic grip with special jaws that apply even pressure on the syringe barrel using the lowest amount of force possible to prevent breakage. The syringe barrel is placed between the jaws of the lower grip and onto the revolver of the upper grip simultaneously, automatically centering the specimen with respect to the test axis.
Self-aligning specimen grip with revolver plate
A revolver plate with various cutouts for different syringe diameters can be rotated at the bottom of the grip until the respective cut-out is in place. The syringe barrel is placed between the jaws of the lower grip and onto the revolver of the upper grip simultaneously, automatically centering the specimen with respect to the test axis. As the crosshead moves upward in the tensile direction, the self-aligning specimen grip positioned under the Luer lock collar pulls the collar upward.
Advantages:
- One tool for a variety of syringe diameters
- Easy to use
- Precise specimen alignment
Annex G4: Luer lock adapter collar torque resistance
Luer fittings are designed to provide leak-free connections between syringes and needles or tubes (e.g., IV tubes). The Luer lock collar conforms to the Luer cone dimensions and is pressed onto the cone and held by friction. A needle hub, for example, is then screwed onto the collar to attach the needle to the syringe. During this attachment process, the Luer lock collar must stay in place to maintain the integrity of the seal. The Luer lock collar torque resistance test is designed to verify whether the collar is able to withstand an applied torque as the needle is attached. The test measures the torque at which the collar starts to rotate on the syringe.
1 Specimen grip with three-finger flange (upper)
2 Pneumatic specimen grip (lower)
Pneumatic specimen grip (lower)
Pneumatic grip with special jaws that apply even pressure on the syringe barrel using the lowest amount of force possible to prevent breakage. The syringe barrel is placed vertically between the jaws of the lower grip, with the tip facing upward, and automatically centered with respect to the test axis.
Specimen grip with three-finger flange (upper)
The three-finger flange is tightened around the Luer lock collar with a manual wheel. The collar is then rotated at a specified speed and torque, and the maximum torque peak, or the torque at which the collar starts to rotate on the syringe, is measured. Specimens either pass or fail the test based on the specifications.
Advantages:
- One fixture for a variety of syringe diameters
- Easy to use
- Precise specimen alignment
Annex G5: Luer lock rigid tip cap unscrewing torque
Luer lock tip caps can come in many variations. The purpose of the tip cap is to provide a seal until the needle or tube (i.e., IV tube) is attached. The tip cap is screwed on to the Luer lock collar and the test is performed to verify whether the tip cap can be removed from the syringe with a reasonable torque.
1 Specimen grip with three-finger flange (upper)
2 Pneumatic specimen grip (lower)
Pneumatic specimen grip (lower)
Pneumatic grip with special jaws that apply even pressure on the syringe barrel using the lowest amount of force possible to prevent breakage. The syringe barrel is placed vertically between the jaws of the lower grip, with the tip facing upward, and automatically centered with respect to the test axis.
Specimen grip with three-finger flange (upper)
The three-finger flange is tightened around the tip cap with a manual wheel. The cap is then rotated at a specified speed and torque, and the maximum torque peak, or the torque at which the tip cap starts to rotate on the syringe, is measured. Specimens either pass or fail the test based on the specifications.
Advantages:
- One fixture for a variety of syringe diameters
- Easy to use
- Precise specimen alignment
Annex G6: Pull-off force of the tip cap or the needle shield
This test is performed on needle shields (needle present) or tip caps (no needle present) that conform to the Luer cone dimensions and are pressed onto the cone and held by friction. The test is designed to verify whether the needle shield or tip cap is able to withstand an axial pull-off force and avoid premature detachment from the syringe barrel. Premature detachment would affect the integrity of the syringe. Annex G6 describes two separate methods:
G6 Method 1
1 Specimen grip with three-finger flange (upper)
2 Pneumatic specimen grip (lower)
Pneumatic specimen grip (lower)
Pneumatic grip with special jaws that apply even pressure on the syringe barrel using the lowest amount of force possible to prevent breakage. The syringe barrel is placed vertically between the jaws of the lower grip, with the tip facing upward, and automatically centered with respect to the test axis.
Specimen grip with three-finger flange (upper)
The three-finger flange is tightened around the needle shield or tip cap with a manual wheel. The upper grip is then moved upward in the tensile direction, and the maximum force required to pull off the needle shield or tip cap is measured and recorded.
Advantages:
- One fixture for a variety of syringe diameters
- Easy to use
- Precise specimen alignment
G6 Method 2
1 Special self-aligning specimen grip (upper)
2 Pneumatic specimen grip (lower)
Pneumatic specimen grip (lower)
Pneumatic grip with special jaws that apply even pressure on the syringe barrel using the lowest amount of force possible to prevent breakage. The syringe barrel is placed between the jaws of the lower grip and onto the revolver of the upper grip simultaneously, automatically centering the specimen with respect to the test axis.
Self-aligning specimen grip with revolver plate
A revolver plate with various cutouts for different syringe diameters can be rotated at the bottom of the grip until the respective cut-out is in place. The syringe barrel is placed between the jaws of the lower grip and onto the revolver of the upper grip simultaneously, automatically centering the specimen with respect to the test axis. As the crosshead moves upward in the tensile direction, the self-aligning specimen grip positioned under the needle shield or tip cap pulls the needle shield or tip cap upward.
Advantages:
- One fixture for a variety of syringe diameters
- Easy to use
- Precise specimen alignment
USP (United States Pharmacopeia) Chapter <1382> and <382>
- ISO 11040-4, ISO 11040-6, and ISO 11040-8 are referenced in the United States Pharmacopeia USP <1382>
The chapter <1382> provides information and guidance to assist in evaluating the functional proficiency of elastomeric components as part of packaging/drug delivery systems intended for parenteral dosage forms. - In addition, ISO 11040-4 and ISO 11040-8 are refrenced in the USP Chapter <381>. Chapter <382> addresses functional proficiency requirements for packaging/drug delivery systems intended for parenteral dosage forms.
Testing system for syringes to ISO 11040
As a manufacturer of materials testing machines, ZwickRoell offers a complete product portfolio for testing to ISO 11040. With interchangeable testing adapters, the solution can be effortlessly adapted to different types of syringes and ensures that testing of prefilled syringes is standardized. The zwickiLine is the ideal single-column testing machine for this application, designed for testing with low forces up to 5 kN.
Traceable, tamper-proof test results in accordance with FDA 21 CFR Part 11
- Ever-increasing demands are placed on software used in the medical and pharmaceutical industries to document the traceability of completed actions.
- With the traceability option, testXpert III enables logging of all actions and changes before, during and after the test, making test results and the documentation traceable and protecting them from manipulation.
- Integrated user management and functions such as electronic records and electronic signature ensure that test results are always protected from tampering.
- Together with the organizational measures and procedure instructions that apply to the individual companies themselves, the requirements of FDA in 21 CFR Part 11 are fulfilled.
- ZwickRoell also offers a qualification service package (DQ/IQ/OQ) for validation support.
- testXpert III logs all test and system related actions and settings and can therefore always answer the question “When does who do what, why and who is responsible?”
Learn more about the testXpert III
Traceability option.
Downloads
- Product Information: ISO 11040-4/-6 Annex C1 - Flange Breakage Resistance PDF 2 MB
- Product Information: ISO 11040-4/-6 Annex C2 - Luer Cone Breakage Resistance PDF 571 KB
- Product Information: ISO 11040-4/-6 Annex F - Needle Penetration Test PDF 136 KB
- Product Information: ISO 11040-4/-6 Annex G1 - Needle Pull-Out Force (Needle Bond Strength) PDF 2 MB
- Product Information: ISO 11040-4/-6 Annex E / G2 - Glide Force Test / Closure System Liquid Leakage Test PDF 263 KB
- Product Information: ISO 11040-4/-6 Annex G3 - Luer Lock Adapter Collar Pull-Off Force PDF 3 MB
- Product Information: ISO 11040-4 Annex G4 - Luer Lock Adapter Collar Torque Resistance PDF 1 MB
- Product Information: ISO 11040-4 Annex G5 - Luer Lock Rigid Tip Cap Unscrewing Torque PDF 3 MB
- Product Information: ISO 11040-4/-6 Annex G6, Method 1 - Pull-Off Force of Needle Shield (Tip Cap) PDF 4 MB
- Product Information: ISO 11040-4/-6 Annex G6, Method 2 - Pull-Off Force of Needle Shield (Tip Cap) PDF 3 MB
- Industry Brochure: medical industry PDF 6 MB
- Product Information: Traceable and Reliable Test Results in Accordance with FDA 21 CFR Part 11 PDF 1 MB
- Industry Brochure: Biomechanics PDF 8 MB
Frequently asked questions on syringe testing
Syringe testing is performed to assess the integrity and functionality of prefilled syringes. It is regulated by strict international standards (ISO 11040) and is critical for safe dispensing of medications, anticoagulants and biologics. To meet the strict requirements of the standard, the testing machine to be used must be equipped with a wide variety of interchangeable test fixtures that can accommodate a multitude of syringe types.
A prefilled syringe (PFS) is a disposable needle-based injection system pre-filled with a specified substance to be administered. This device was developed in an effort to provide innovative delivery solutions for the increasing use of self-administrated therapies.
There are a variety of syringe types available on the market. Prefilled syringes can be made of glass or plastic, with or without needle:
- Prefilled glass syringes are still predominant, partly based on long-term experience with this material. However, plastic prefilled syringes are on the rise due to advancements in polymer materials that have increased their biocompatibility with other syringe components.
- Needle-free prefilled syringes are commonly used for vaccines and typically include a Luer lock attachment for the needle, some type of tamper-resistant element, and an elastomer stopper on the nozzle of the syringe. Prefilled syringes with staked-in needle are typically used for biologic and pharmaceutical drugs. This type of syringe has the needle already attached.