Test fixtures for natural and artificial biomaterials
In the development of implants, the characteristic value determination of natural biomaterials is also required.
Load test on human femur Bending test on sheep bones Fatigue test on blood vessels Various specimen grips Evaluation
Load test on human femur with strain gauges applied
The test is used to establish to what extent an implanted endoprosthesis stiffens the bone, thereby causing a stress protection effect of the bone. For this a human femur is placed in a ZwickRoell 20 kN AllroundLine. combined with a bearing, intended to eliminate transverse forces. The femur head is then loaded axially. Strain gauges adhered to the surface of the bone enable comparison of the surface stress on the femur before and after implantation of the prosthesis.
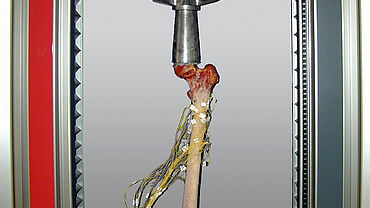

Flexure tests on sheep bones
A 3-point flexure test on sheep bones was designed to determine flexural strength after a fracture has healed. For this, the bone that has healed after a fracture is fixed or cast at its ends in supports. The load is applied using a zwickiLine 0.5 kN table-top testing machine. The specimen grips are designed so that rotation of the bone by defined angular degrees is possible, allowing the flexural strength of the entire area of the bone to be determined. The characteristic values obtained in this way are used in an FEM simulation of the healing behavior of the bone fracture.
Evaluation of mechanical characteristics (compression and creep strength) of scaffolds in tissue engineering
Research of biomaterials requires that the test environment corresponds to actual conditions as much as possible. For this reason, testing in this field is preferably carried out in fluid baths or complete incubators. In this application, cells from bone marrow are applied to a carrier material and cultivated in a liquid nutrient under cyclic mechanical stimulation.
This also requires an ambient temperature of 37 °C, 100% humidity and cyclic loading (using a ZwickRoell testing machine with an electromechanical actuator) under very low forces. The load is applied to the specimen via a die integrated in the incubator from above and the specimens immersed in the liquid nutrient are flushed with CO2 or N2 to adjust the pH value. Extensions lie between 30 and 100 μm.
The testing of knee replacement material is simulated with bone cells in scaffolds through cyclic loads with a strain greater than =1 µm in-vitro. These bone replacement substrates are used to promote fracture healing or tissue and bone cell growth on implants, preventing the need to remove bone material from other areas of the patient's skeletal structure.








