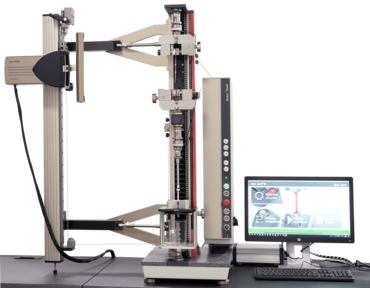
Cleanroom-Compatible Testing Machines
Materials testing in cleanrooms in the pharmaceutical, medical and biomedical industries is subject to strict requirements in terms of particle count per m3. These include, in particular, the requirement to reliably rule out risks of contamination from harmful substances or germs. Specific requirements for testing in cleanrooms and associated controlled environments are described in standards ISO 14644 1:2016-06; ISO 14644-14 and VDI 2083 Page 9.1. As a manufacturer of materials testing machines, ZwickRoell offers cleanroom-compatible testing machines that meet the strict requirements for testing in cleanrooms.
Standards and guidelines for materials testing in cleanrooms ISO 14644 requirements and cleanroom classifications Cleanroom-compatible materials testing machines Applications Certificate FAQ Request a consultation
Standards and guidelines for materials testing in cleanrooms
Standard | Title | Content |
| ISO 14644 1:2016-06 | Cleanrooms and associated controlled environments - Part 1: Classification of air cleanliness by particle concentration (ISO 14644-1:2015); German version EN ISO 14644-1:2015 | Table 1 specifies the particle concentration at various particle sizes within the nine ISO classifications. The use of Table 1 ensures that the particle size ranges appropriated for different classes are better defined. |
| ISO 14644-14 | Cleanrooms and associated controlled environments - Part 14: Assessment of suitability for use of equipment by airborne particle concentration (ISO 14644-14:2016); German version EN ISO 14644-14:2016 | This standard combines the classification of air cleanliness based on the particle concentration of the cleanroom with the suitability of equipment for use in cleanrooms and associated controlled environments. This standard specifies a methodology for evaluating the cleanroom suitability of equipment (for example, machines, measuring instruments, processing equipment, components, tools) for use in cleanrooms and associated controlled environments with respect to airborne particle cleanliness as specified in ISO 14644-1. |
| VDI 2083 Page 9.1 | Cleanroom technology - Compatibility with required cleanliness and surface cleanliness | This guideline provides information on planning, generating, maintaining, restoring and verifying the cleanliness of equipment and air handling units. (...) Requirements, procedures and detection methods therefore depend on the specific use of the cleanroom and the possible contaminants. The guideline includes a standardized procedure for the qualification of equipment and air handling units for cleanliness controlled environments. |
Overview of cleanroom classes to ISO 14644-1
Maximum permissible particle count per m3
| Class | 0.1 μm | 0.2 μm | 0.3 μm | 0.5 μm | 1.0 μm | 5.0 μm |
| ISO 1 | 10 | 2 | - | - | - | - |
| ISO 2 | 100 | 24 | 10 | 4 | - | - |
| ISO 3 | 1,000 | 237 | 102 | 35 | 8 | - |
| ISO 4 | 10,000 | 2,370 | 1,020 | 352 | 83 | - |
| ISO 5 (requirements met by zwickiLine Z2.5 TN+) | 100,000 | 23,700 | 10,200 | 3,520 | 832 | 29 |
| ISO 6 (requirements met by zwickiLine) | 1,000,000 | 237,000 | 102,000 | 35,200 | 8,320 | 293 |
| ISO 7 | - | - | - | 352,000 | 83,200 | 2,930 |
| ISO 8 | - | - | - | 3,520,000 | 832,000 | 29,300 |
| ISO 9 | - | - | - | 35,200,000 | 8,320,000 | 293,000 |
Our solutions for materials testing in cleanroom environments
ZwickRoell is the first manufacturer of cleanroom-compatible testing machines that meet the strict requirements for materials testing in cleanroom environments. The zwickiLine materials testing machine was further developed to meet these requirements. In a force range of 0.5 kN to 2.5 kN, all Class 6 cleanroom requirements are met; the zwickiLine Z2.5 TN+ additionally meets all Class 5 cleanroom requirements.
- The materials test can be carried out directly in the cleanroom, eliminating the need to transfer specimens from the cleanroom to the testing lab.
- It is possible to integrate the testing machine directly into the production process in the cleanroom (e.g., test on insulin pens).
- Biological specimens can be tested without the risk of contamination.
All relevant tests can be conveniently performed with the powerful, flexible, yet cost-effective zwickiLine testing machine. With its wide range of equipment options, the zwickiLine meets all the necessary requirements while producing reliable test results.
Advantages of the cleanroom-compatible zwickLine Z2.5 TN+ materials testing machine
The technical properties of the cleanroom-compatible materials testing machine zwickiLine Z2.5 TN+ significantly differentiate it from the zwickiLine. It provides sophisticated possibilities for applications with increased test requirements.
- A powerful wear-free AC motor enables a high crosshead speed of 3000 mm/min over the entire force range up to 2.5 kN.
- High resolution of the crosshead movement of 0.95 nm and superb speed accuracy with 18 µm/min, deliver reliable test results even at very slow test speeds and short test travel distances in the µm range.
- The stiffness is four times higher than for a regular zwickiLine Z2.5, which also allows for testing of very stiff specimens without an additional extensometer.
Differentiating features of the zwickiLine Z0.5, Z1.0, Z2.5 vs. zwickiLine Z2.5 TN+
Differentiating features | zwickiLine | zwickiLine Z2.5 TN+ |
| crosshead speed | Z0.5/1.0: 0.0005 ... 2000 mm/min* Z2.5: 0.0005 ... 1000 mm/min* | 3000 mm/min* over the entire force range up to 2.5 kN |
| Resolution of the crosshead movement | Z0.5: 0.0830 μm Z1.0: 0.0554 μm Z2.5: 0.0277 μm | 0.95 nm |
| Stiffness | 1 | 4x higher than the zwickiLine Z2.5 |
* Values apply to machines with closed safety door and closed safety guard in automatic mode and to machines without safety device and/or without safety guard. For machines with the safety door and/or safety guard open, the speed is reduced to 600 mm/min.
testXpert testing software and the traceability option
Results from materials testing in cleanroom environments are transferred to the testXpert testing software. With the traceability option integrated in the software, the regulations of FDA 21 CFR Part 11 can be met according to the ZwickRoell white paper.
ZwickRoell is not just an excellent partner for testing in cleanrooms, we also serve many other areas in the medical and pharmaceutical industry.
Cleanroom-compatible materials testing machine applications
Testing in cleanrooms is particularly important in the medical and pharmaceutical industries. Cleanrooms are required, among other areas, in medical research, medical engineering and in the aseptic production of drugs in the pharmaceutical industry. For example, in the manufacture of implants, drug coated medical devices, pharmaceutical products and sterile medicines such wound care materials and bandages, cleanrooms are mandatory.
Important cleanroom tests in the field of therapy systems:
- Autoinjector spring simulation. Spring forces have a significant influence on the success of injections using an autoinjector
- Quality assurance tests on insulin pens and carpules to ISO 11608-1, ISO 11608-2, and ISO 11608-3
- Syringe testing to ISO 11040 with parts -4, -6 and -8 is critical for safe dispensing of medications, anticoagulants and biologics
- The breakaway force and glide force are important parameters used in the selection of suitable syringes and carpules.
- Determination of the plunger glide force on sterile single-use syringes made from plastic and other materials used for medical purposes, with fluid contents (water) to EN ISO 7886-1 Annex E (2017)
- Stability test of a Luer or Lure lock connector using various tests according to standards ISO 80369-7 and ISO 80369-20
- Serial and parallel tests on syringe systems
- Break resistance of injection needles to ISO 9626
Important cleanroom tests in the field of catheters and stents:
- Coefficient of friction of cardiovascular and urological catheters
- Tensile test on catheter systems to DIN EN ISO 10555
- Flexure test on guide wires, catheters, and tubes
- Test on stents that are subject to strong stresses during insertion and retention in the blood vessel
Important cleanroom tests in the field of medical packaging:
- Peelability of dimensionally stable or rigid packaging
- Compression test on vial lids (referenced in USP Chapter 1207)
- Turning torque of screw caps
- Breakage resistance of snapoof ampoules
- Secondary and transport packaging made of paper and cardboard
- Determination of the separating force of pharmaceutical capsule shells
- Manufacturing and testing of micro needles (case example)
Available certificates for cleanroom-compatible materials testing machines from ZwickRoell
- Confirmation of cleanroom compatibility, air purity class 6 to ISO14644-1:2016, ISO 14644-14 and VDI 2083-9.1, incl. certificate for zwickiLine Z0.5, Z1.0 and Z2.5. The cleanroom compatibility of the zwickiLine has been verified on the Z0.5, Z1.0 and Z2.5. Since all the machines in the zwickiLine series Z0.5, Z1.0 and Z2.5 are identical in construction, we can also guarantee cleanroom suitability for the other construction heights.
- Confirmation of cleanroom compatibility, air purity class 5 to ISO14644-1:2016, ISO 14644-14 and VDI 2083-9.1, incl. certificate for zwickiLine Z2.5 TN+. Cleanroom compatibility of the zwickiLine has been verified on a Z2.5 TN+.

Frequently asked questions on cleanroom testing
Standards relevant for materials testing in cleanroom environments are ISO 14644 1:2016-06 Cleanrooms and associated controlled environments - Part 1:Classification of air cleanliness by particle concentration , ISO 14644-14 Cleanrooms and associated controlled environments - Part 14:Assessment of suitability for use of equipment by airborne particle concentration as well as guideline VDI 2083 Page 9.1 "Cleanroom technology - Compatibility with required cleanliness and surface cleanliness.
A cleanroom is a room that has a very low concentration of airborne particles. Airborne particles are all particles and substances that float in the air and can usually not be seen with the naked eye. The requirements for cleanrooms and associated controlled environments are described in standard EN ISO 14644.
Cleanrooms are required, among other areas, in medical research, medical engineering and in the aseptic production of drugs in the pharmaceutical industry. Clean rooms are also important in semiconductor manufacturing, in optics and laser technology, and in the field of aerospace engineering. In the manufacture of implants, drug coated medical devices, pharmaceutical products and sterile medicines such as wound care materials and bandages, clean rooms are mandatory.
Applications of cleanroom-compatible materials testing machines involve important tests in the field of therapy systems, e.g., quality assurance tests on insulin pens and carpules to ISO 11608-1, ISO 11608-2, and ISO 11608-3; in the area of catheters and stents, e.g., tensile testing of catheter systems to DIN EN ISO 10555; and in the field of medical packaging, for example compression testing of vial lids (referenced in USP Chapter 1207).